
( 1.5) that the volume is chosen so that it is neither so small that it has no chance of containing a representative selection of molecules nor so large that (in the case of gases) changes of pressure cause significant changes of density throughout the volume. In which the notation “” is consistently used to indicate the dimensions of a quantity. The density ρ of a fluid is defined as its mass per unit volume and indicates its inertia or resistance to an accelerating force. Table 1.1 Specific Gravities, Densities, and Thermal Expansion Coefficients of Liquids at 20° C

However, if highly accurate values are needed, particularly at extreme conditions, then specialized information should be sought elsewhere.
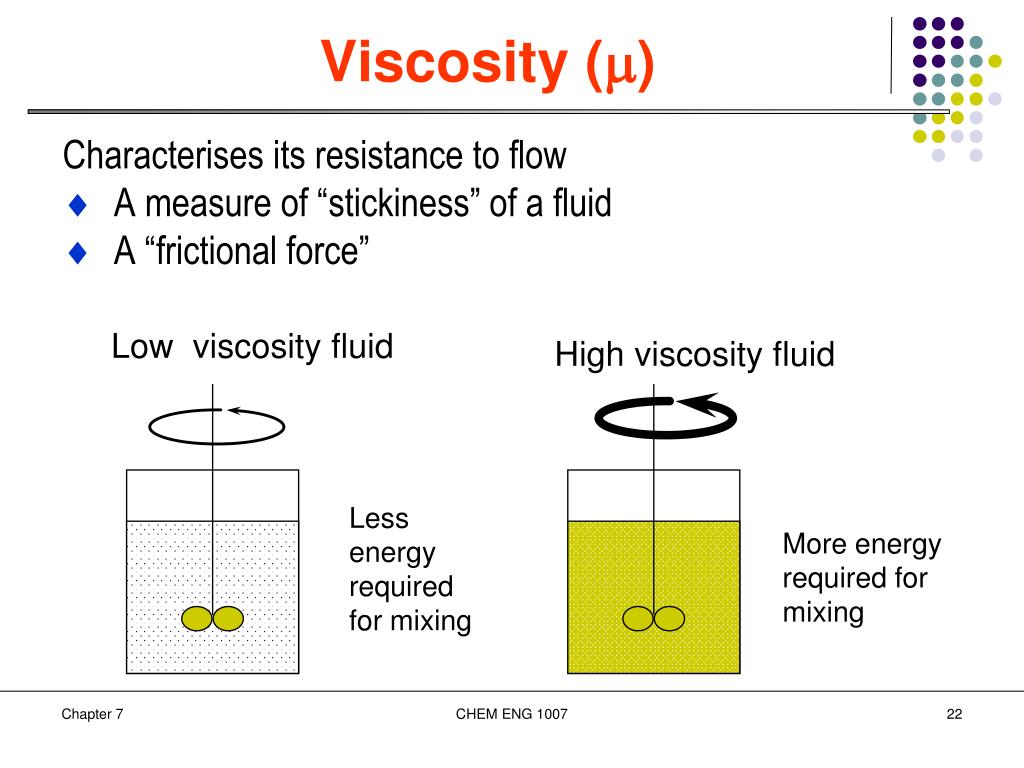
( 1.9)–( 1.11) for an explanation of the specific gravity and coefficient of thermal expansion columns.) The accuracy of the values given in Tables 1.1–1.6 is adequate for the calculations needed in this text. Representative densities of liquids are given in Table 1.1. For liquids, density depends primarily on the particular liquid and, to a much smaller extent, on its temperature. Density depends on the mass of an individual molecule and the number of such molecules that occupy a unit of volume. Except in certain special cases, such as the flow of a compressible gas (in which the density is not constant) or a liquid under a very high shear rate (in which viscous dissipation can cause significant internal heating), or situations involving exothermic or endothermic reactions, we shall ignore any variation of physical properties with pressure and temperature.ĭensity. Typical processes often run almost isothermally, and in these cases the effect of temperature can be ignored. The density of gases depends almost directly on the absolute pressure for most other cases, the effect of pressure on physical properties can be disregarded. For liquids, viscosity also depends strongly on the temperature for gases, viscosity is approximately proportional to the square root of the absolute temperature. The physical properties depend primarily on the particular fluid. Each of these will be defined and viewed briefly in terms of molecular concepts, and their dimensions will be examined in terms of mass, length, and time (M, L, and T). There are three physical properties of fluids that are particularly important: density, viscosity, and surface tension. Learn More Buy 1.4 Physical Properties-Density, Viscosity, and Surface Tension

Fluid Mechanics for Chemical Engineers: with Microfluidics, CFD, and COMSOL Multiphysics 5, 3rd Edition


 0 kommentar(er)
0 kommentar(er)
